- A clean break
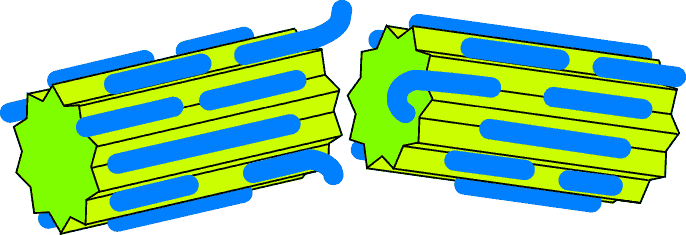
A clean break
Here the two crystals part company as they would "normally" do, and continue
growing separately.
- Attachment
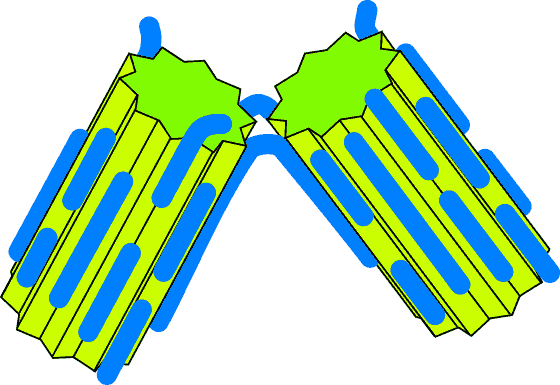
Attachment
Here the two crystals remain joined at a "hinge" - perhaps eventually coming to
join along their entire length. The chances of this sort of thing happening
depend on the relative sizes of various forces - and it is probably not a very
likely outcome.
- Single unzip
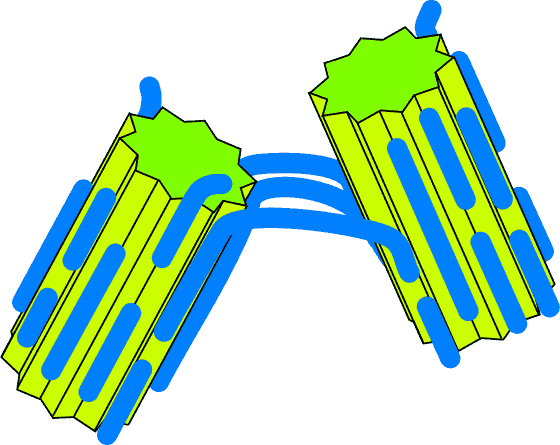
Single unzip
More likely is an "unzipping" process. Here the bond between the polysaccharide
and the clay is weaker than the bonds between individual monosaccharide units -
and as a consequence when force is applied at either end the polysaccharide
comes "unzipped" from one of the crystals.
- Double unzip

Double unzip
Another sort of "unzipping" is theoretically possible: the "double unzip".
This works because the force applied at the crystal-polymer junction depends on
the angle between them. The more obtuse the angle, the greater the force. The
result is that the two sides of the zip keep approximately in step with one
another.
A true double zip is not very likely to happen in practice. It only arises in a
relatively narrow range of conditions.
The event depends to some extent on whether the unzipping occurs in a single
process, or in a series of jerks.
If the event is more-or-less continuous, the "single unzip" seems the most
likely outcome.

